Abstract
Introduction
After allogeneic cell transplantation (HCT) for acute myeloid leukemia (AML) relapse remains the main cause of morbidity and mortality. Relapse rates directly translate into patients' overall survival. The connection between early human cytomegalovirus (CMV) replication and reduced leukemic recurrence has first been established by this group (Elmaagacli et. al. Blood 2011), using a pp65-antigenemia assay and confirmed by Green et al. (Blood 2013), Takenaka et al. (BBMT 2015) and others. In a more recent study by Teira et al. (Blood 2016) using PCR to detect reactivation, this effect has been questioned and the controversy remains probably due to methodological differences between studies.
Methods
Patients with AML received allogeneic HCT at the Department of Bone Marrow Transplantation of the University Hospital Essen between 10/1997 and 10/2017. All patient-, donor-, HCT- and virology data was prospectively documented and retrospectively analyzed. The immunosuppressive regimen consisted of cyclosporine A and methotrexate. The conditioning was according to physician's choice and included only non-anti-thymocyte globuline (ATG) conditioning regimens. Early supportive and follow-up care was identical for all patients. Patients were followed for up to 60 months. Surviving patients were censored at last follow-up date. Competing risk analysis was performed for calculating cause-specific relapse-incidences with non-relapse mortality as competing risk. CMV titers were measured at the Department of Virology using quantitative PCR (qPCR) and a pp65 antigenemia assay. CMV reactivation was defined as a replication of >500 CMV copies per ml EDTA blood or as >25 pp65 antigen expressing cells per 5×105 white blood cells. If several CMV reactivation episodes occurred, only the interval to the first episode after HCT was analyzed.
Results
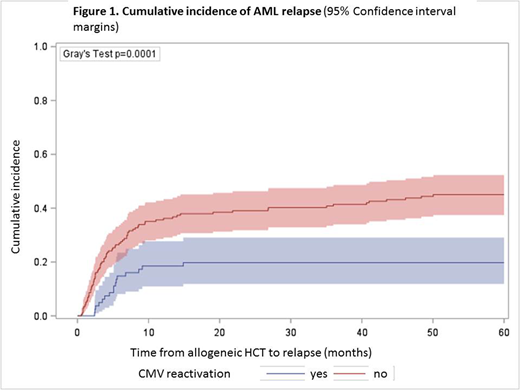
A total of 420 consecutive adult patients (median age, 48 years; range, 16-73 years) with AML and HCT were included for analysis. Over 60 months of follow-up, cumulative incidence of CMV reactivation was detected in 28% of patients and AML relapsed in 34%. In this cohort, the sensitivity of qPCR or pp65 detection methods did not differ (p=0.35) with regard to the incidence of CMV reactivation. CMV reactivation associated with significantly reduced AML relapse risk. The relapse incidence (RI) for patients with CMV reactivation was 0.18 (95% Confidence interval (CI), 0.12-0.25) compared to 0.41 (95% CI, 0.35-0.46) for patients without CMV reactivation (p<0.0001). This significant difference was reproduced for prognostic AML subgroups in first complete remission (RI, 0.14 with CMV reactivation versus 0.35 without; p=0.02) and for higher risk AML (RI, 0.20 versus 0.45; p<0.0001). Notably, donor-recipient serostatus constellation significantly (p=0.005) associated with AML relapse reduction, independently of CMV reactivation. CMV positive donors had a RI of 0.40 (95% CI, 0.33-0.46) compared to 0.28 in CMV negative donors (95% CI, 0.22-0.34).
Discussion
This new PCR dataset confirms previous reports (Elmaagacli et al. 2011; Takenaka et al. 2015) of an independent reduction of AML relapse risk after CMV reactivation, using qPCR detection in a homogeneous population of AML patients. While the association between CMV reactivation and early relapse (< day+100) has also been confirmed by Green et al. (Blood 2013) and relapse (>day +100) by Takenaka et al. (BBMT 2015), our data shows that late relapse (>24 months) may occur in patients without CMV reactivation. While the aforementioned studies exclusively used pp65 antigenemia titers as a determinant of CMV reactivation, Teira et al. (Blood 2016) analyzed PCR samples. The sensitivity of CMV reactivation using PCR or pp65 antigen titers was identical in our dataset. With regard to the on-going controversy on the role of CMV reactivation and AML relapse, this data shows a reduction of relapse in the quantitative PCR age.
Turki:Neovii Biotech: Other: subsidies for the costs of travel. Beelen:Medac: Consultancy, Other: Travel Support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal